
APA Style
Sunday M. Ajayi, Samuel O. Olusanya, Sunday F. Abimbade, Alex E. Didunyemi, Michael O. Atunde, Dele P. Fapojuwo, Emmanuel G. Olumayede, Olayide S. Lawal, Cecilia O. Akintayo, Dosu. Malomo. (2024). Preparation and Characterization of Acetate Cellulose Laurate Ester in Sodium Acetate/Zinc Chloride Systems. Biomaterials Connect, 1 (Article ID: 0003). https://doi.org/10.69709/BIOMATC.2024.171176MLA Style
Sunday M. Ajayi, Samuel O. Olusanya, Sunday F. Abimbade, Alex E. Didunyemi, Michael O. Atunde, Dele P. Fapojuwo, Emmanuel G. Olumayede, Olayide S. Lawal, Cecilia O. Akintayo, Dosu. Malomo. "Preparation and Characterization of Acetate Cellulose Laurate Ester in Sodium Acetate/Zinc Chloride Systems". Biomaterials Connect, vol. 1, 2024, Article ID: 0003, https://doi.org/10.69709/BIOMATC.2024.171176.Chicago Style
Sunday M. Ajayi, Samuel O. Olusanya, Sunday F. Abimbade, Alex E. Didunyemi, Michael O. Atunde, Dele P. Fapojuwo, Emmanuel G. Olumayede, Olayide S. Lawal, Cecilia O. Akintayo, Dosu. Malomo. 2024. "Preparation and Characterization of Acetate Cellulose Laurate Ester in Sodium Acetate/Zinc Chloride Systems." Biomaterials Connect 1 (2024): 0003. https://doi.org/10.69709/BIOMATC.2024.171176.
 ACCESS
Research Article
ACCESS
Research Article
Volume 1, Article ID: 2024.0003

Sunday M. Ajayi
ajayi2016victor@gmail.com

Samuel O. Olusanya

Sunday F. Abimbade

Alex E. Didunyemi

Michael O. Atunde

Dele P. Fapojuwo

Emmanuel G. Olumayede

Olayide S. Lawal

Cecilia O. Akintayo

Dosu. Malomo
1 College of Agriculture, Science and Information Technology, Venite University, KM 2, Ayetoro-Road, Iloro-Ekiti, Nigeria
2 Department of Chemistry, Federal University Oye-Ekiti, P.M.B. 373, KM 3 Oye-Are Road, Oye-Ekiti, Nigeria
3 Department of Chemistry, Nelson Mandela University, P.O. Box 77000, Gqeberha 6031, South Africa
* Author to whom correspondence should be addressed
Received: 01 Oct 2024 Accepted: 16 Oct 2024 Available Online: 17 Oct 2024 Published: 24 Oct 2024
The objective of this study was to explore the preparation and characterization of acetate cellulose laurate ester in sodium acetate/zinc chloride systems. The cellulose used in the study was obtained from oil palm empty fruit bunch and oil palm frond. The characterization of both the isolated cellulose and the acetate cellulose laurate ester was carried out using Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), Differential thermogravimetric (DTG), and Thermogravimetric analysis (TGA). The FT-IR analysis of the acetate cellulose laurate ester revealed the presence of C=O, confirming its modification. The crystallinity of the isolated cellulose and the acetate cellulose laurate ester was found to be 67–72% and 51–54%, respectively. The thermal stability of the isolated and modified cellulose esters was 360–340 ℃ and 380–365 ℃, respectively. The solubility test showed that the prepared acetate cellulose laurate esters were soluble in different solvents. However, this study found that the synthesis of acetate cellulose laurate ester in an aqueous medium is not feasible. The improved hydrophobic character, good thermal stability, water retention value, and excellent solubility in different solvents of the acetate cellulose laurate ester reported in this study make it a potential material for applications in bioplastics, and packaging materials.
Cellulose is a renewable natural polymer material that can be found in all plants, including oil palm waste like oil palm empty fruit bunches and oil palm fronds [1]. The oil palm is a tropical plant that thrives in warm climates at elevations below 500 meters above sea level and requires at least 1.5 meters of spacing above ground level [2]. Oil palm empty fruit bunches are devoid of fruits, while palm fronds contain oil palm leaves [3]. Studies have shown that oil palm empty fruit bunches (OPEFB) and oil palm fronds (OPF) are lignocellulosic sources that can be used as raw materials for cellulose production [4]. Cellulose and its derivatives, such as cellulose esters, are used in numerous industries to produce cellulose-related products like foods, plastics, films, and pharmaceutical products [5]. Cellulose is a linear and semi-crystalline polysaccharide that consists of repeating anhydroglucose units (AGUs) linked by β-1,4-glycosidic bonds [6,7]. As the campaign against environmental degradation continues, cellulose has been identified as an alternative to petroleum, and its use for producing many new materials has increased geometrically due to its economic merits such as abundance, biodegradability, low cost, sustainability, renewability, nontoxicity, ease of modification, and biocompatibility [8]. Despite these advantages, cellulose cannot be used directly for many applications due to some drawbacks, such as insolubility in water and some organic solvents. This is primarily due to the intra- and inter-molecular hydrogen bonds within its polymer structure.. Additionally, the poor thermal plasticity of unmodified cellulose makes it unsuitable for compression moulding applications [8]. However, physical and chemical modification methods such as esterification and etherification can be used to alter the physicochemical properties of cellulose significantly [8]. Cellulose esters with long-chain residues of fatty acid chlorides are potential materials for bio-plastic applications due to their mechanical properties [9,10]. Previous studies have reported on the preparation of cellulose laurate ester through esterification using lauric acid as the esterifying agent [11,12,13]. Recently, Xiaoxiang et al. [14] reported the synthesis of cellulose laurate by transesterification in an AmimCl/DMSO cosolvent system and 1,8-Diazabicyclo [5.4.0] undec-7-ene (DBU) as a catalyst using vinyl laurate as an acylation agent. Schenzel et al. [15] have also reported the synthesis of cellulose laurate through the transesterification method using 1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) as a catalyst. Long-chain cellulose esters have been prepared by many researchers, but the negative impact of the spent reagents on the environment and the high cost of the reagents used have been noted [16,17,18,19,20,21]. The introduction of acetate groups, in addition to the laurate groups, onto the cellulose chain can potentially enhance the physicochemical properties of cellulose derivatives products [16,22]. This necessitates the need for an environmentally friendly and cost-effective method for preparing acetate cellulose laurate ester. Therefore, this study presents a simple method of preparing acetate cellulose laurate ester using a sodium acetate/zinc chloride system. Sodium acetate/zinc chloride system has low toxicity and requires no acid scavengers. Moreover, this study successfully demonstrated the extraction of cellulose from oil palm waste using this method.
2.1. Materials The chemical reagents used are sodium hydroxide (>99%), anhydrous sodium sulphite (>97% purity), nitric acid (70% purity), hydrochloric acid (36% purity), potassium hydroxide (>98% pure), ethanol and methanol (99% purity), lauroyl chloride (>98% purity), sodium hypochlorite 3.5% w/v, sulphuric acid (98% purity), anhydrous zinc chloride (AR, Kermel, 98%), toluene (>98%). All the reagents are purchased from Sigma-Aldrich and AR Karmel and used as purchased. The cellulose used in this study was extracted from oil palm empty fruit bunch (OPEFB) and oil palm frond (OPF) according to a method described in our previous studies (see supplementary section) [2,4]. The proximate analysis of the OPEFB and OPF has also been reported in the previous studies [2,4]. The isolated cellulose from oil palm empty fruit bunch and oil palm frond were denoted ICB, and ICF, respectively. 2.2. Methods 2.2.1. Preparation of Acetate Cellulose Laurate Ester in an Aqueous Medium To gain insight into the behaviour of cellulose-lauroyl chloride in water, lauroylation in an aqueous environment was performed based on the method outlined in our previous studies [2,4] with minor adjustments. The activation of ICB or ICF (1.0 g) was initiated by NaOH (20 mL; 0.5 M) in a 500 mL beaker. The cellulose-NaOH mixture was mechanically stirred at 300 rpm for 10 minutes at 30 ± 2 °C using a KJJ-1 fixed-time power mixer (model KJJ-1 60 W). Lauroyl chloride (5 mL) was added gradually to the activated cellulose using a burette while continuous stirring was maintained on a magnetic stirrer (model hotplate 78-1) at 30 ± 2 °C. The solution was maintained in an alkaline region by carefully adding NaOH (0.5M). The product was filtered and washed with ethanol (15 mL) three times, followed by distilled water three times. The product was further washed with ethanol three times and acetone three times and dried at 50 °C for 48 h using a thermostat oven (model DHG-9101-OSA). ICF was treated similarly. 2.2.2. Preparation of Acetate Cellulose Laurate Ester in Sodium Acetate/Zinc Chloride Medium To initiate the activation process of cellulose, 20 mL of sodium acetate (1 M) was added to ICB (1.0 g) in a pre-dried round flat-bottom flask (model DHG-9101-OSA) at 60 °C for 8 h using a hotplate stirrer (model hotplate 78-1) at 250 rpm. The mixture was stirred at 30 ± 2 °C for 20 minutes, after which anhydrous zinc chloride (1 wt%) was added and stirred for 15 minutes at 50 °C. Subsequently, lauroyl chloride (5 mL) was slowly added to the activated cellulose mixture using a burette while continuously stirring. Once all the lauroyl chloride was added, the solution was refluxed at 90 °C for 45 minutes. The mixture was then poured into 50 mL of ethanol to precipitate the acetate cellulose laurate ester. The precipitated product was filtered using vacuum filtration and washed with ethanol (15 mL) three times and acetone (30 mL) five times. The acetate cellulose laurate ester (ACLB-5) was dried at 60 °C for 24 h using the aforementioned drying equipment. Further, the process was repeated with ICF to obtain ACLF-5. 2.2.3. Determination of Degree of Substitution of Acetate Cellulose Laurate Ester To determine the degree of substitution (DS) of the acetate cellulose laurate ester, the titrimetric method described by Lawal et al. [23] with minor modifications was employed. One gram of each sample (ACLB-5 or ACLF-5) that had been dried at 60 °C for 24 h was dissolved in 1% aqueous sodium chloride and titrated with NaOH (1 M) using a phenolphthalein indicator. The titration was stopped when the phenolphthalein indicator’s color permanently disappeared, indicating the endpoint. The DS was calculated using the following Equation (1) The corrected weight of modified cellulose (wc) is given by Equation (2). 2.2.4. Proximate Analysis of Isolated Cellulose and Acetate Cellulose Laurate Ester Proximate analysis (moisture, ash, crude fibre, and crude fat contents) of isolated cellulose (ICB, ICF) and acetate cellulose laurate ester (ACLB-5 and ACLF-5) was determined according to standard AOAC methods (AOAC, 1996) without modification.
3.1. Fourier Transform Infrared Spectroscopy (FT-IR) Fourier Transform Infrared (FTIR) spectra of isolated cellulose and acetate cellulose laurate ester were recorded by a PerkinElmer Spectrum 100 (PerkinElmer, Waltham, MA, USA) using the KBr pellet procedure for both samples. Before the measurement, the KBr tablets were dried at 105 °C for 3 h to get rid of moisture. 3.2. Crystallinity The crystallinity of the native cellulose and acetate cellulose laurate ester was investigated by a D-8 Advance instrument operated at 40 mA and voltage 45 kV at 25 °C with Cu Kα. The measurement was recorded at an angle 2θ value between 5 to 50° at a scan speed of 3.00°/min. 3.3. Thermal Stability The thermal stability of both samples was characterized by using TGA/DTG on a QG500 thermogravimetric analyzer. About 2–3 mg of samples were heated from 20 to 800 °C under a nitrogen atmosphere at a heating rate of 10 °C/min. 3.4. Water Retention Value Measurement Water retention value (WRV) is an important method of evaluating the hydrophobic behaviour of cellulosic materials relative to moisture absorption. The water retention value was determined according to the method reported in our previous studies [2,4].
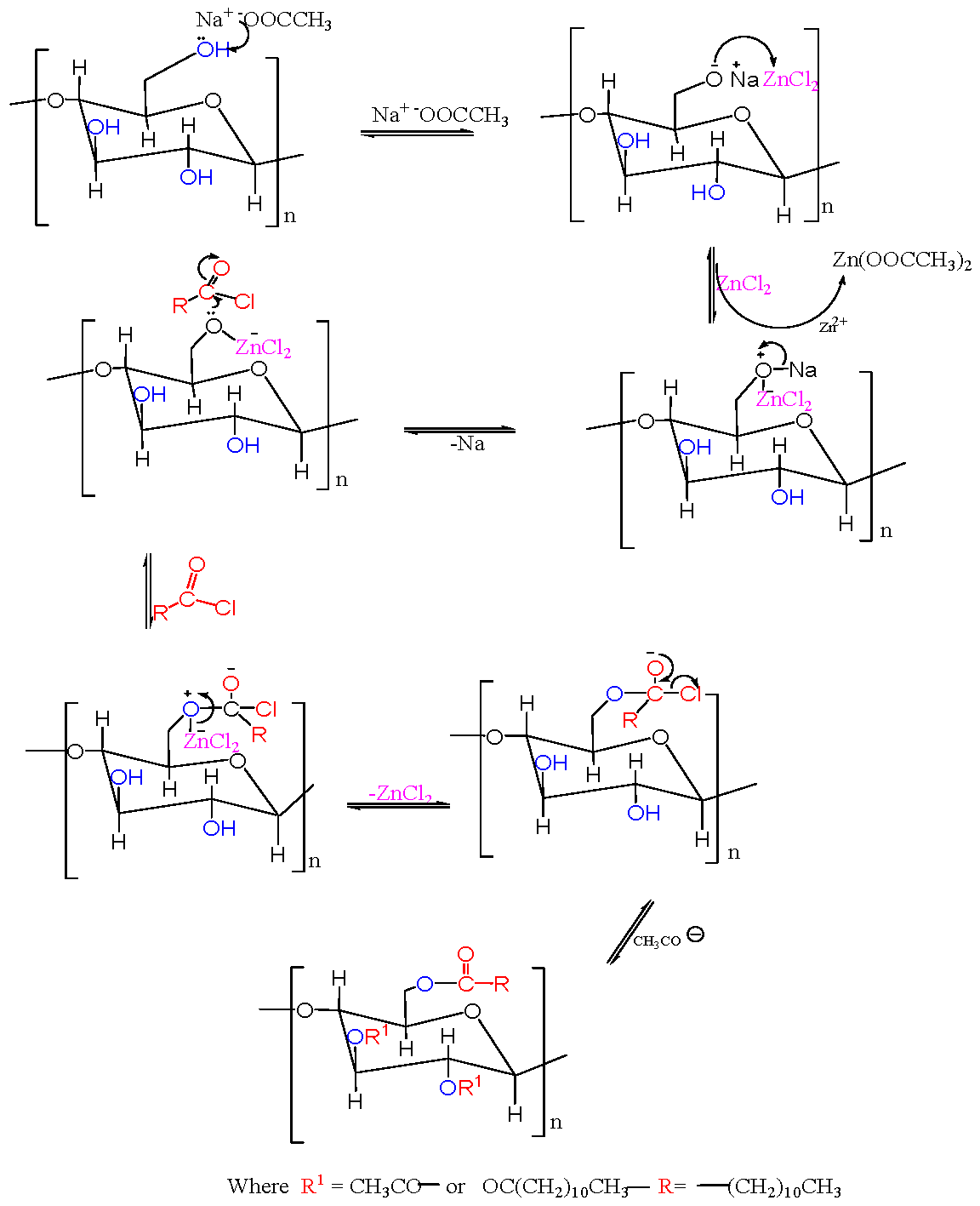
4.1. Preparation of Acetate Cellulose Laurate Ester in an Aqueous Medium The preparation of acetate cellulose laurate ester in an aqueous medium provided valuable information about the behaviour of native cellulose in long-chain fatty acid chlorides. Specifically, it was observed that lauroyl chloride was decomposed into its corresponding carboxylic acid by water molecules, leading to the rupture of the fibrillar architecture of cellulose during activation with sodium hydroxide, lauroyl chloride, and its corresponding carboxylic acid. This resulted in the absorption of the compounds into the cellulose matrix, causing excessive swelling. It suggests that acid hydrolysis occurs between lauroyl chloride and sodium hydroxide, which is detrimental to the esterification reaction. When the reaction temperature exceeded 40 °C, cellulose degradation occurred, leading to browning, which is consistent with previous findings by Jérôme et al. [24]. Therefore, the preparation of acetate cellulose laurate ester in an aqueous medium is not feasible. The flowchart of the extraction of cellulose from oil palm waste, as previously reported in our studies, is shown in Figure S1. It has been previously reported that modifying cellulose with long-chain fatty acids in NaOH/ethanol solution as non-acidic catalysts at high temperatures is not effective [24]. This study suggests that a lauroyl chloride-water-NaOH cosolvent would be an appropriate solvent for achieving the maximum swelling of cellulose. 4.2. Preparation of Acetate Cellulose Laurate Ester in Sodium Acetate/Zinc Chloride Medium The cellulose, whether it be ICB or ICF, was activated by sodium acetate. The mixture’s pH was stabilized by the formation of acetic acid and the presence of unreacted sodium acetate, which acted as a buffer. The addition of zinc chloride and the reaction medium increased the nucleophilic properties of the cellulose surface and the electrophilic character of the alpha carbon atom of lauroyl chloride, respectively. Under these conditions, lauroyl chloride underwent a substitution reaction at the expense of hydrolysis. The reaction between ZnCl2 and sodium acetate resulted in the formation of an acetyl group, whichreacted with the cellulose-hydroxyl groups available through nucleophilic attack during esterification [16,17]. The reaction mechanism of cellulose with lauroyl chloride in a sodium acetate/zinc chloride system is depicted in 4.3. Proximate Analysis of Isolated Cellulose and Acetate Cellulose Laurate Esters The outcome of the proximate analysis of isolated cellulose (ICB and ICF) and acetate cellulose laurate ester (ACLB-5 and ACLF-5) is depicted in Table 1. The findings revealed that the moisture content of the acetate cellulose laurate esters was lower than that of unmodified cellulose, which may be attributed to the increased hydrophobic character of acetate cellulose laurate ester compared to its native form. Additionally, the observed decrease in the percentage ash content of acetate cellulose laurate ester relative to unmodified cellulose might be due to the loss or erosion of the mineral content inherent in the cellulose during modification. Similarly, the reduction in ash content of ACLB-5 (1.62 ± 0.020%) and ACLF-5 (1.71 ± 0.031%), as well as the decrease in fiber and fat contents of ACLB-5 and ACLF-5 compared to isolated cellulose (ICB and ICF), was attributed to the effect of slight degradation resulting from the esterification process. Proximate analysis of isolated cellulose and acetate cellulose laurate ester. 4.4. Effect of Preparation Method on the Degree of Substitution The degree of substitution was determined using Equation (1), which showed that ACLF-5 and ACLB-5 had average degrees of substitution of 0.6 and 0.9, respectively. As the reaction time and molar ratio of AGU to lauroyl chloride was increased while keeping other reaction conditions constant, the degree of substitution also increased. Willberg-Keyrilainen and Jarmo previously reported a DS of 0.9 for cellulose laurate ester made using a homogeneous method in LiCl/DMAc solution and pyridine at 80 °C for 16 h or 100 °C for 5 h [26]. Xiaoxiang et al. also found that the DS of cellulose laurate ester increased from 1.47 to 2.41 with higher reaction temperatures (70–120 °C) and reached 2.63 with a molar ratio of 1:12 [14]. Willberg-Keyrilainen and Jarmo also reported that the DS of homogeneously esterified cellulose ester increased from 0.3 to 1.3 depending on the length of the side chain [26]. Schenzel et al. reported a DS value of 0.4 for long-chain cellulose esters made using 1,5,7-triazabicyclo [4.4.0] dec-5-ene (TBD) as a catalyst at 115 °C for 24 h [15]. The use of zinc chloride shielded the hydroxyl groups from the bulkiness of lauroyl chloride, which increased electron density and promoted effective interaction with the alpha carbon atom of lauroyl chloride, leading to a steadily increased DS value.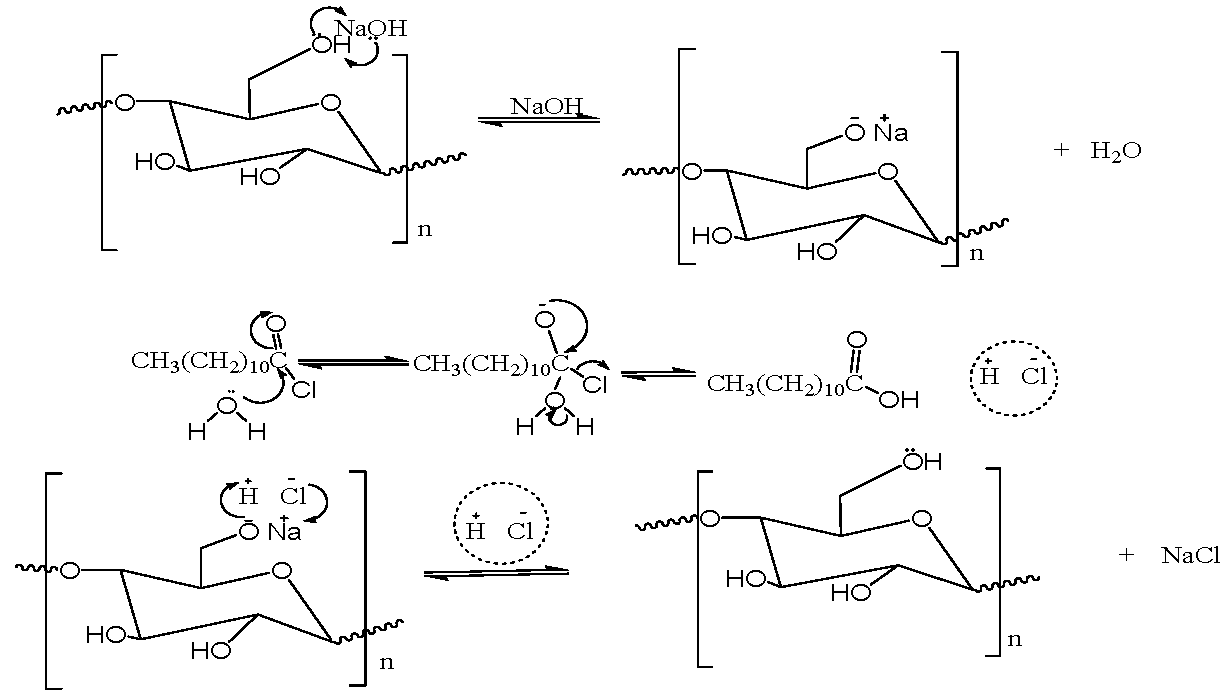
Samples
Moisture Content
Ash Content
Crude Fibre
Crude Fat
(%)
(%)
(%)
(%)
ICB
6.06 ± 0.27
2.0 ± 0.16
6.96 ± 0.10
0.89 ± 0.02
ICF
5.82 ± 0.13
3.04 ± 0.02
7.01 ± 0.03
0.68 ± 0.05
ACLB-5
3.85 ± 0.03
1.62 ± 0.02
3.14 ± 0.03
0.32 ± 0.04
ACLF-5
3.81 ± 0.01
1.71 ± 0.03
3.51 ± 0.02
0.29 ± 0.01
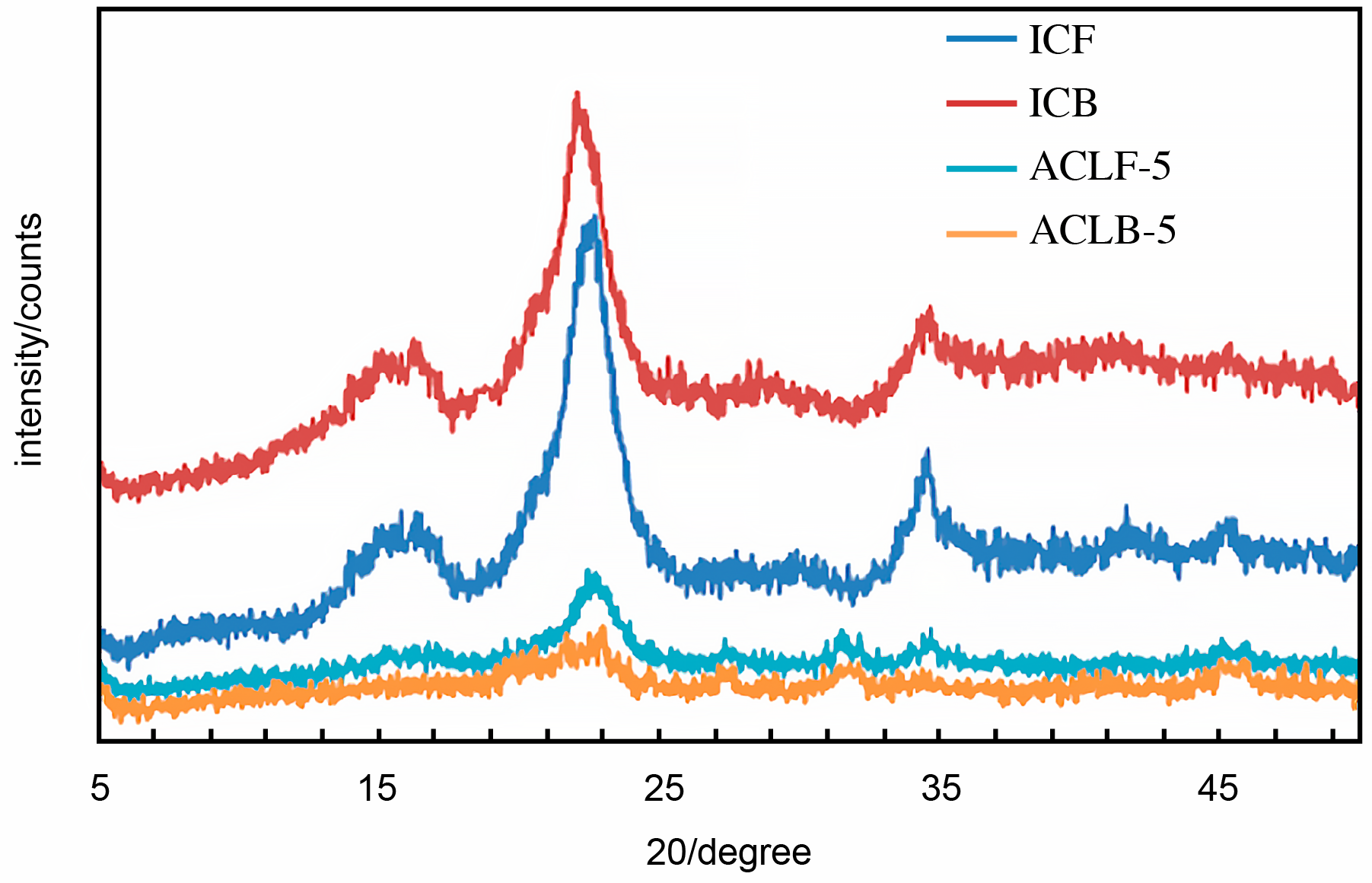
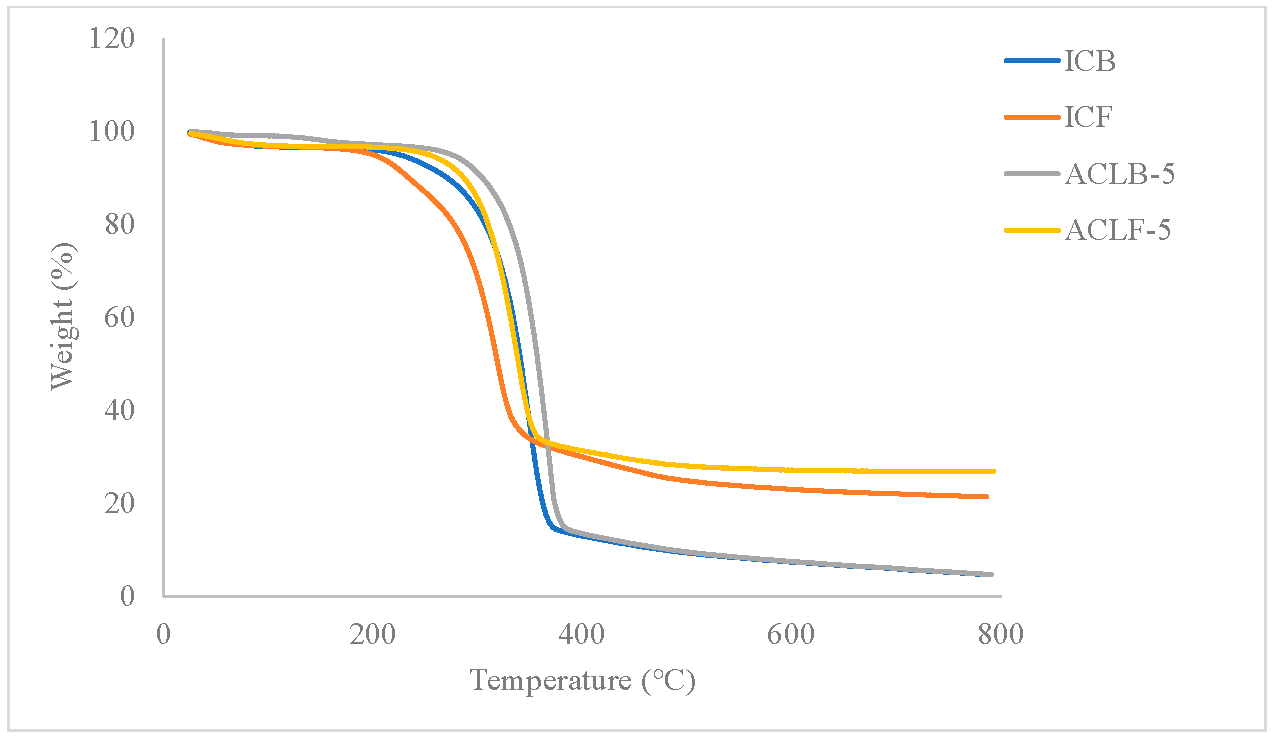
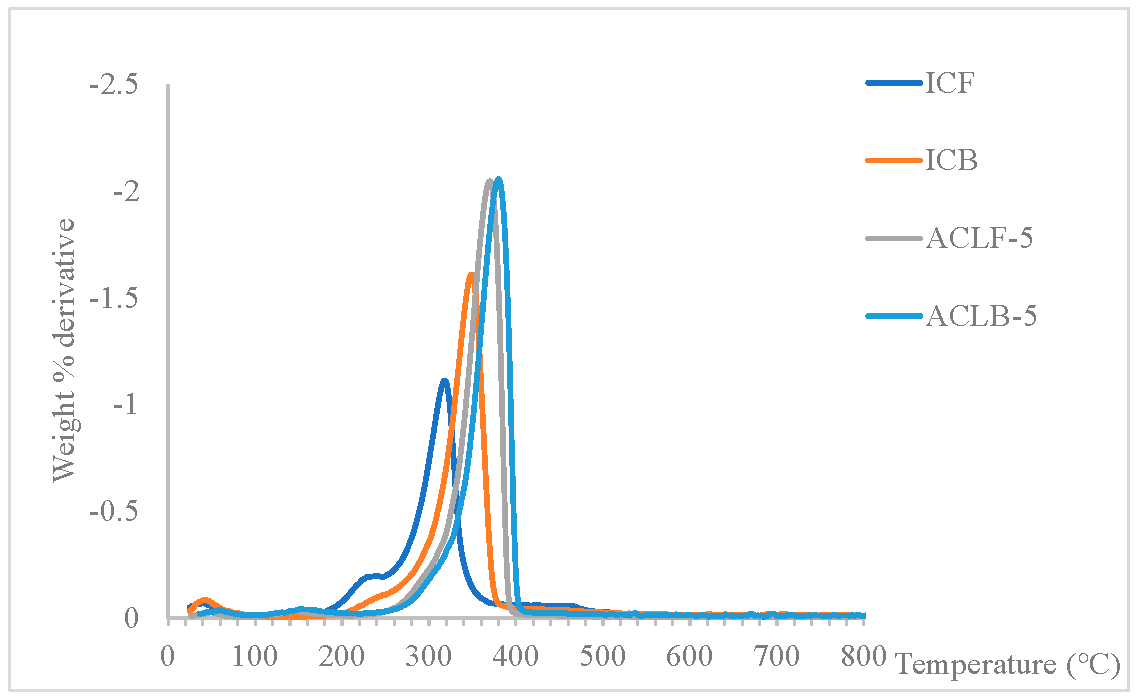
5.1. Fourier Transform Infrared Spectroscopy (FT-IR) The infrared spectra of acetate cellulose laurate ester and isolated cellulose are depicted in Figure 1. The peak observed in the range of 3250–3600 cm−1 was assigned to vO–H stretching vibration resulting from hydrogen bonding [4]. The peak in the range of 2950–2800 cm−1 was identified as C–H (methyl; asymmetry), while the absorption peaks at 1614 cm−1 and 1655 cm−1 in the ICB and ICF spectra, respectively, were attributed to water molecules inherent in the cellulose fiber capillary. Additionally, the peak in the range of 1420–1376 cm−1 was assigned to CH2 bending vibration. The weak peaks in the range of 1250–1130 cm−1 and at 1050 cm−1 in all the samples may be attributed to δCH2 and C–O–C asymmetric stretch vibration, respectively [27]. The noticeable peaks with a weak pointed shoulder in the range of 950–860 cm−1 in all the samples were attributed to C1–O–C of β-(1–4)-glycosidic linkages stretching vibration in cellulose I. The appearance of the peak in the range of 1720–1740 cm−1 in acetate cellulose laurate esters (ACLB-5 and ACLF-5) spectra was attributed to C=O ester stretching, revealing the preparation of ACLB-5 and ACLF-5. The absence of an absorption peak at 1800 cm−1 in the spectra indicated that the products are free of any unreacted acid chloride. 5.2. X-Ray Diffraction (XRD) The X-ray diffraction (XRD) patterns of acetate cellulose laurate ester and unmodified cellulose are shown in Figure 2. The Scherrer crystallite size of ICB and ACLB-5 were 1.654 ± 0.0399 nm and 0.978 ± 0.0526 nm, and ICF and ACLF-5 were 1.179 ± 0.0313 nm, and 0.948 ± 0.0463 nm, respectively. These values are the average crystal dimensions of the cellulose particles. The crystallinity index of ICB, ACLB-5, ICF, and ACLF-5 were 67.22%, 51.66%, 72.18%, and 54.07%, respectively. In this study, the isolated cellulose from oil palm waste was found to have a highly crystalline structure, which is attributed to inter- and intra-molecular hydrogen bonds. This finding is consistent with previous literature. [28,29]. In comparison, the low crystallinity of acetate cellulose laurate esters (58.62%) compared to cellulose benzoate ester (61.38%) prepared in an aqueous medium, as reported in our previous studies [2,4], may be attributed to an increased amorphous character resulting from the introduction of an acetate group into the cellulose backbone, which accounts for the broadness of ACLB-5 and ACLF-5 diffractogram peak at 2θ = 21–24° [14]. 5.3. Thermogravimetric Analysis The thermal stability of both isolated cellulose and acetate cellulose laurate esters was investigated using TGA and DTG, and the thermogram curves are shown in Figure 3. As can be seen from the curves, both isolated cellulose and acetate cellulose laurate esters (ACLB-5 and ACLF-5) had initial weight loss (2–8 wt%) below 140 °C, which was due to the evaporation of water inherent in cellulose fiber capillaries. The second step of ICB and ICF decomposition began at 240 °C and 220 °C, respectively, and terminated at 360 °C and 340 °C, respectively. For ACLB-5 and ACLF-5, the second step of decomposition started at 290 °C and 280 °C, respectively, and ended at 380 °C and 360 °C, respectively. The increased thermal stability of ACLB-5 and ACLF-5 confirms the formation of acetate cellulose laurate esters. The introduced long-chain fatty acid group onto the cellulose skeleton might be orderly arranged to form a new ordered structure, which could be accountable for the enhanced thermal stability [9,30]. Additionally, the increased amorphous character could be attributed to the presence of acetyl groups formed through esterification, as indicated by the XRD analysis. The cellulose degradation occurred via dehydration, depolymerization, and glucosan formation, leading to the cleavage of 1,4 glycosidic bonds [31]. The pyrolysis residues (char) at 800 °C were likely due to a high carbon content from the bonded acetyl group and lauroyl chloride [32]. The Differential Thermogravimetric (DTG) curves indicated that the degradation of ACLB-5 and ACLF-5 occurred at regions 380 °C and 365 °C, respectively, with the evolution of volatile products [33], which supported the TGA result. The degree of heat intensity associated with the acetate cellulose laurate esters, as shown by the DTG (Figure 4), could be due to the cumulative effect of acetyl groups and long-chain hydrocarbon residues. 5.4. Hydrophobicity To test the extent of hydrophobicity and hydrophilicity of the acetate cellulose laurate ester and unmodified cellulose, respectively, water retention value (WRV) was used. Water retention value gives information on the penetration of water into the interior parts of the cellulose fibers via the capillary system. The WRV of ICB and ICF decreased from 128 ± 1.009% to 113 ± 1.018% and 125 ± 1.672% to 110 ± 0.948%, respectively, as reported in our previous studies [2,4]. The high WRV of ICB and ICF was due to their hydrophilic properties. The WRV of ACLB-5 and ACLF-5 were 52 ± 1.046% and 55 ± 1.004%, respectively. The low WRV of ACLB-5 and ACLF-5 signified a high degree of hydrophobization, which agreed with other analysis. The long aliphatic chains are hydrophobic while cellulose is hydrophilic. Thus, the higher the aliphatic side chains attached to the cellulose backbone or chain, the higher the hydrophobicity of the modified cellulose. It was noted that variables such as surface area, weight of samples, and particle size do not significantly affect the WRV, which agreed with [4,34]. 5.5. Solubility The study investigated the solubility of acetate cellulose laurate ester in various solvents. Most of the previously prepared cellulose laurate esters were found to be completely insoluble in tetrahydrofuran (THF), DMSO, and N-Dimethyformamide (DMF). However, the acetate cellulose laurate esters obtained in this study were found to be completely soluble in chloroform, partially soluble in THF, DMSO, and DMF, and completely insoluble in acetone, ethanol, and methanol. The extent of solubility may depend on the degree of substitution (DS). The findings suggest that the solubility of acetate cellulose laurate ester in THF, DMSO, and DMF enhances its industrial potential for the production of numerous cellulosic-based products such as films, tissue engineering, bioplastics, and packaging materials.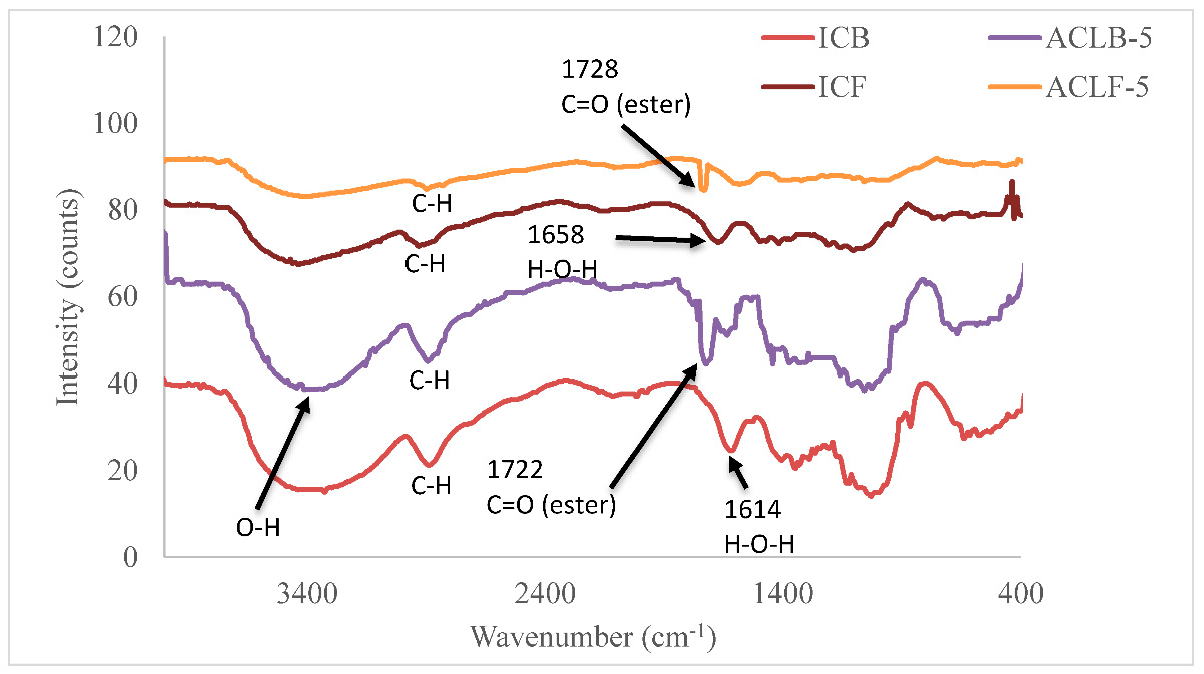
In conclusion, a novel method of introducing both acetyl group and long-chain fatty acid onto cellulose polymer chain simultaneously was explored. The study also examined the effect of the reaction medium and solubility. The results revealed that the sodium acetate/zinc chloride system is an excellent method of introducing both acetyl and long-chain fatty acid groups onto the cellulose backbone simultaneously. The benefits of this system include a short reaction time, ease of separation, and reduced environmental degradation caused by co-solvents. The improved hydrophobic character, high thermal stability, moderate water retention value, and excellent solubility in various solvents indicate that acetate cellulose laurate ester is a promising material for bioplastics, packaging, food, drug delivery, and pharmaceutical applications.
| ICB | Isolated cellulose from oil palm empty fruit bunch |
| ICF | Isolated cellulose from oil palm frond |
| ACLB-5 | Acetate cellulose laurate ester from oil palm bunch |
| ACLF-5 | Acetate cellulose laurate ester from oil palm frond |
| WRV | Water retention value |
| AOAC | Association of Official Analytical Chemists |
| DMSO | Dimethylsulfoxide |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| TBD | 1,5,7-triazabicyclo [4.4.0] dec-5-ene |
| DS | degree of substitution |
| LiCl | lithium chloride |
| DMAc | Dimethylacetamide |
| AGU | anhydroglucose units |
| XRD | X-ray diffraction |
| TGA | Thermogravimetric Analysis |
| DTG | Differential Thermogravimetric |
| THF | tetrahydrofuran |
| DMF | N-Dimethyformamide. |
S.M.A.: Writing—original draft, Formal analysis, Investigation, and Methodology; S.O.O.: Validation, Investigation, and Conceptualization; S.F.A.: Data analysis and validation; A.E.D.: Investigation and methodology; M.O.A.: Methodology and investigation; D.P.F.: Resources and Validation; E.G.O.: Validation and Supervision; O.S.L.: Validation and Supervision; C.O.A.: Validation and Supervison; D.M.: Validation and Supervision.
All data and figures analyzed during this study are included in this article (or available in the cited references).
Not applicable.
The authors have no competing interests to declare that are relevant to the content of this article.
No funding was received for conducting this study.
Not applicable.
Download the Supplementary data to this article.
[1] K.J. Edgar, C.M. Buchanan, J.S. Debenham, P.A. Rundquist, B.D. Seiler, M.C. Shelton, et al., "Advances in cellulose ester performance and application" Prog. Polym. Sci., vol. 26, pp. 1605-1688, 2001. [Crossref]
[2] S.M. Ajayi, S.O. Olusanya, K.O. Sodeinde, A.E. Didunyemi, M.O. Atunde, D.P. Fapojuwo, et al., "Hydrophobic modification of cellulose from oil palm empty fruit bunch: Characterization and application in Pickering emulsions stabilization" Carbohydr. Polym. Technol. Appl., vol. 5, p. 100282, 2023. [Crossref]
[3] H.P.S. Abdul Khalil, A.H. Bhat, M. Jawaid, P. Amouzgar, R. Ridzuan, M.R. Said, "Agro-Wastes: Mechanical and physical properties of resin impregnated oil palm trunk core lumber" Polym. Compos., vol. 31, pp. 638-644, 2010. [Crossref]
[4] S.M. Ajayi, S.O. Olusanya, K.O. Sodeinde, E.G. Olumayede, O.S. Lawal, A.E. Didunyemi, et al., "Application of hydrophobically modified cellulose from oil palm frond in Pickering emulsions stabilization" Carbohydr. Polym. Technol. Appl., vol. 4, p. 100248, 2022. [Crossref]
[5] L. Brinchi, F. Cotana, E. Fortunati, J.M. Kenny, "Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications" Carbohydr. Polym., vol. 94, pp. 154-169, 2013. [Crossref]
[6] M.Y. Eliza, M. Shahruddin, J. Noormaziah, W.D. Wan Rosli, "Carboxymethyl cellulose (CMC) from oil palm empty fruit bunch (OPEFB) in the new solvent dimethyl sulfoxide (DMSO)/tetrabutylammonium fluoride (TBAF)" J. Phys. Conf. Ser., vol. 622, pp. 1-10, 2015. [Crossref]
[7] M. Ioelovich, "Models of supramolecular structure and properties of cellulose" J. Polym. Sci. Ser. A, vol. 58, pp. 925-943, 2016. [Crossref]
[8] C. Chen, M. Cho, B.W. Kim, J.D. Nam, Y. Lee, "Thermo plasticization and characterization of kenaf fiber by benzoylation" J. Ind. Eng. Chem., vol. 18, pp. 1107-1111, 2012. [Crossref]
[9] X. Cao, X. Peng, L. Zhong, S. Sun, D. Yang, X. Zhang, et al., "A novel transesterification system to rapidly synthesize cellulose aliphatic esters" Cellulose, vol. 21, pp. 581-594, 2014. [Crossref]
[10] P. Willberg-Keyrilainen, H. Orelma, J. Ropponen, "Injection molding of thermoplastic cellulose esters and their compatibility with poly(lactic acid) and polyethylene" Materials, vol. 11, 2018. [Crossref]
[11] L. Crépy, L. Chaveriat, J. Banoub, P. Martin, N. Joly, "Synthesis of cellulose fatty esters as plastics-influence of the degree of substitution and the fatty chain length on mechanical properties" ChemSusChem Chem. Sustain. Energy Mater., vol. 2, pp. 165-170, 2009. [Crossref] [PubMed]
[12] L. Crépy, V. Miri, N. Joly, P. Martin, J.M. Lefebvre, "Effect of side chain length on structure and thermomechanical properties of fully substituted cellulose fatty esters" Carbohydr. Polym., vol. 83, pp. 1812-1820, 2011. [Crossref]
[13] F.-Y. Huang, X.J. Wu, Y. Yu, Y.H. Lu, "Preparation and properties of cellulose laurate (CL)/starch nanocrystals acetate (SNA) bio-nanocomposites" Polymers, vol. 7, pp. 1331-1345, 2015. [Crossref]
[14] X. Wen, H. Wang, Y. Wei, X. Wang, C. Liu, "Preparation and characterization of cellulose laurate ester by catalyzed transesterification" Carbohydr. Polym., vol. 168, pp. 247-254, 2017. [Crossref] [PubMed]
[15] A. Schenzel, A. Hufendiek, C. Barner-Kowollik, M.A.R. Meier, "Catalytic transesterification of cellulose in ionic liquids: Sustainable access to cellulose esters" Green Chem., vol. 16, pp. 3266-3271, 2014. [Crossref]
[16] H. Daisuke, W.K. Samuel Budi, I. Daiki, W. Naoki, T. Kenji, "Direct one-step synthesis of a formally fully biobased polymer from cellulose and cinnamon flavor" Green Chem., vol. 21, pp. 4927-4931, 2019. [Crossref]
[17] H. Daisuke, W.K. Samuel Budi, N. Shuhei, Y. Makoto, Y. Yoshiro, K. Ryohei, et al., "Effect of anion in carboxylate-based ionic liquids on catalytic activity of transesterification with vinyl esters and the solubility of cellulose" RSC Adv., vol. 9, pp. 4048-4053, 2019. [Crossref]
[18] N.O. Kelechukwu, G. Stephane, G. Etienne, C. Henri, A.R.M. Michael, "Critical Review on Sustainable Homogeneous Cellulose Modification: Why Renewability Is Not Enough" ACS Sustain. Chem. Eng., vol. 7, pp. 1826-1840, 2019. [Crossref]
[19] R. Kakuchi, R. Ito, S. Nomura, H. Abroshan, K. Ninomiya, T. Ikai, et al., "Mechanistic insight into the organocatalytic properties of imidazolium-based ionic liquids and a positive co-solvent effect on cellulose modification reactions in an ionic liquid" RSC Adv., vol. 7, pp. 9423-9430, 2017. [Crossref]
[20] L.P. Hinner, J.L. Wissner, A. Beurer, B.A. Nebel, B. Hauer, "Homogeneous vinyl ester-based synthesis of different cellulose derivatives in 1-ethyl-3-methylimidazolium acetate" Green Chem., vol. 18, pp. 6099-6107, 2016. [Crossref]
[21] S. Kohler, T. Liebert, M. Schobitz, J. Schaller, F. Meister, W. Gunther, et al., "Interaction of ionic liquids with polysaccharides 1. Unexpected acetylation of cellulose with 1-ethyl-3-methylimidazolium acetate" Macromol. Rapid Commun., vol. 28, pp. 2311-2317, 2007. [Crossref]
[22] Z.Y. Chen, J.M. Zhang, P. Xiao, W.G. Tian, J. Zhang, "Novel thermoplastic cellulose esters containing bulky moieties and soft segments" ACS Sustain. Chem. Eng., vol. 6, pp. 4931-4939, 2018. [Crossref]
[23] O.S. Lawal, M. Lechnerc, W. Kulicke, "Single and multi-step carboxymethylation of water yam (Disocorea alata) starch: Synthesis and characterization" Int. J. Biol. Macromol., vol. 42, pp. 429-435, 2008. [Crossref]
[24] P. Jérôme, G. Samuel, V.-G. Carlos, B. Marie-Elisabeth, "Long chain cellulose esters with very low DS obtained with non-acidic catalysts" Cellulose, vol. 13, pp. 95-103, 2006. [Crossref]
[25] H. Winkler, W. Vorwerg, H. Wetzel, "Synthesis and Properties of fatty acid starch esters" Carbohydr. Polym., vol. 98, pp. 208-216, 2013. [Crossref]
[26] P. Willberg-Keyrilainen, R. Jarmo, "Evaluation of esterification routes for long chain cellulose esters" Heliyon, vol. 5, p. e02898, 2018. [Crossref]
[27] X. Colom, F. Carrillo, F. Nogue, P. Garriga, "Structural analysis of photodegraded wood by means of FTIR spectroscopy" J. Polym. Degrad. Stab., vol. 80, pp. 543-549, 2003. [Crossref]
[28] L.N. Megashah, H. Ariffin, M.R. Zakaria, M.A. Hassan, "Properties of Cellulose Extract from Different Types of Oil Palm Biomass" Mater. Sci. Eng., vol. 368, p. 012049, 2018. [Crossref]
[29] F.A. Syamani, A. Suryani, "Changes in Oil Palm Frond Fiber Morphology, Cellulose Crystallinity and Chemical Functional Groups during Cellulose Extraction" Chem. Mater. Res., vol. 7, pp. 105-114, 2015. Available online: https://api.semanticscholar.org/CorpusID:55767670.
[30] F.Y. Huang, "Thermal properties and thermal degradation of cellulose tri-Stearate (CTs)" Polymers, vol. 4, pp. 1012-1024, 2012. [Crossref]
[31] A. Adewuyi, V.P. Fabiano, "Isolation and Characterization of Cellulose from Underexploited Golden Melon Skin" Makara J. Sci., vol. 22, pp. 121-126, 2018. [Crossref]
[32] N.G.V. Fundador, Y. Enomoto-Rogers, A. Takemura, T. Iwata, "Syntheses and characterization of xylan esters" Polymers, vol. 53, pp. 3885-3893, 2012. [Crossref]
[33] B. Xiao, R.F. Sun, R.C. Sun, "Chemical, structural and thermal characterizations of alkali soluble lignins and hemicelluloses and cellulose from maize stems, rye straw and rice straw" Polym. Degrad. Stab., vol. 74, pp. 307-319, 2001. [Crossref]
[34] K.L. Kato, R.E. Cameron, "Structure-property relationships in thermally aged cellulose fibers and paper" J. Appl. Polym. Sci., vol. 74, pp. 1465-1477, 1999. [Crossref]
We use cookies to improve your experience on our site. By continuing to use our site, you accept our use of cookies. Learn more